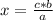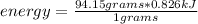Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
The heat of vaporization for ethanol is 0.826 kJ/g. Calculate the heat energy in joules required to...
Questions

Physics, 16.11.2020 22:50

History, 16.11.2020 22:50

Mathematics, 16.11.2020 22:50


Mathematics, 16.11.2020 22:50

Social Studies, 16.11.2020 22:50

Business, 16.11.2020 22:50

Mathematics, 16.11.2020 22:50


History, 16.11.2020 22:50

Business, 16.11.2020 22:50

History, 16.11.2020 22:50

Mathematics, 16.11.2020 22:50

Mathematics, 16.11.2020 22:50



Health, 16.11.2020 22:50

Business, 16.11.2020 22:50

Computers and Technology, 16.11.2020 22:50

History, 16.11.2020 22:50