
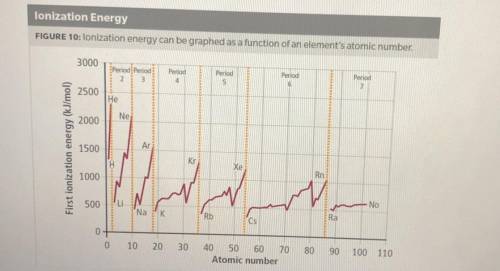
1. How does ionization energy change with atomic number? Use evidence from the graph to support your claim.
2. How does ionization energy change across a period and down a group on the periodic table? Use evidence from the graph to support your claim.
3. What describes an effect on ionization energy when moving down a group? Select all correct answers.
A. The ionization energy increases down a group
B. The ionization energy decreases down a group
C. The valence electrons are in energy levels farther from the nucleus
D. The shielding effect is less

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3
You know the right answer?
1. How does ionization energy change with atomic number? Use evidence from the graph to support your...
Questions

Mathematics, 17.09.2021 05:40







History, 17.09.2021 05:40




Mathematics, 17.09.2021 05:40

Health, 17.09.2021 05:40






Mathematics, 17.09.2021 05:40




