
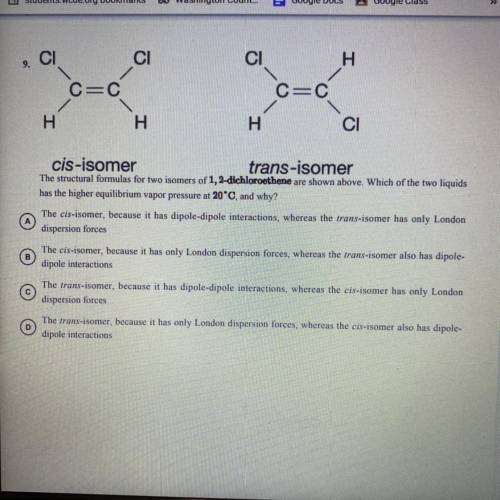
The structural formulas for two isomers of 1, 2-dichloroethene are shown above. Which of the two liquids has the higher equilibrium vapor pressure at 20 degrees Celsius and why?
A) the cis-isomer, because it has dipole-dipole interactions, whereas the trans-isomer has only 1 London dispersion forces.
B) the cis-isomer, because it has only London dispersion forces, whereas the trans-isomer also has dipole-dipole interactions
C) the trans-isomer, because it has dipole-dipole interactions, whereas the cis-isomer has only 1 London dispersion forces
D) the trans-isomer, because it has only 1 London dispersion forces, whereas the cos-isomer also has dipole-dipole interactions

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2


Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
The structural formulas for two isomers of 1, 2-dichloroethene are shown above. Which of the two liq...
Questions

Mathematics, 12.10.2020 05:01


Medicine, 12.10.2020 05:01

Physics, 12.10.2020 05:01


Mathematics, 12.10.2020 05:01

Mathematics, 12.10.2020 05:01



Physics, 12.10.2020 05:01









Mathematics, 12.10.2020 05:01

Mathematics, 12.10.2020 05:01



