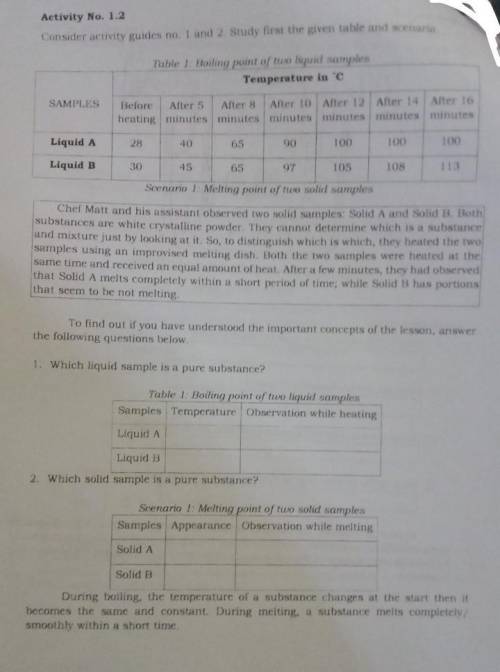
Activity No. 1.2
Consider activity guides no 1 and 2 study first the given table and scenario
...

Activity No. 1.2
Consider activity guides no 1 and 2 study first the given table and scenario
SAMPLES
Table 1 Hoiling point of tuo liquid samples
Temperature in C
Before After 5 After 8 After 10 After 12 After 14 Alter 16
heating minutes minutes minutes minuten minutes minutes
00
100 100 100
Liquid A
07
105
108
Liquid D
Scenario 1 Melting pom of two solid samples
Cher Matt and his assistant observed two solid samples: Solid A and Solid D. Both
substances are white crystalline powder. They cannot determine which is a substance
and mixture just by looking at it. So, to distinguish which is which they heated the two
samples using an improvised melting dish. Both the two samples were heated at the
same time and received an equal amount of heat. Aner a few minutes, they had observed
that Solid A melts completely within a short period of time, while Solid B has portions
that seem to be not melting
To find out if you have understood the important concepts of the lesson, answer
the following questions below
1. Which liquid sample is a pure substance?
Table 1: Boiling point of two liquid samples
Samples Temperature Observation while heating
Liquid A
Liquid B
2. Which solid sample is a pure substance?
Scenano l: Melting point of two solid samples
Samples Appearance Observation while melting
Solid A
Solid B
During boiling, the temperature of a substance changes at the start then it
becomes the same and constant. During melting, a substance melts completely
smoothly within a short time

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 11:00
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow.part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer.o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part.h2o is a stronger acid than oh–, so the equilibrium lies to the right.h2o is a weaker acid than oh–, so the equilibrium lies to the left.h2o is a stronger acid than oh–, so the equilibrium lies to the left.h2o is a weaker acid than oh–, so the equilibrium lies to the right.part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer.ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-(aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left.part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer.no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part.hno2 is a stronger acid than h2o, so the equilibrium lies to the right.hno2 is a weaker acid than h2o, so the equilibrium lies to the left.hno2 is a stronger acid than h2o, so the equilibrium lies to the left.hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

You know the right answer?
Questions


Mathematics, 13.11.2020 23:00


Arts, 13.11.2020 23:00



Chemistry, 13.11.2020 23:00



English, 13.11.2020 23:00

History, 13.11.2020 23:00

Biology, 13.11.2020 23:00


Social Studies, 13.11.2020 23:00


Biology, 13.11.2020 23:00

Mathematics, 13.11.2020 23:00


Chemistry, 13.11.2020 23:00



