
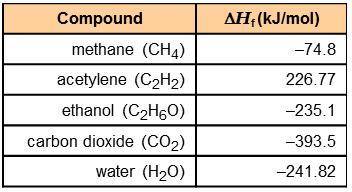
Using the information in the table to the right, calculate the enthalpy of combustion of each of the following substances:
acetylene:
ethanol:
The combustion of 0.25 mol of an unknown organic compound results in the release of 320 kJ of energy. Which of the compounds in the table could be the unknown compound?
ANSWERS:
1.
acetylene: -1,256 kJ/mol
ethanol: -1,277 kJ/mol
2.
ethanol
I already know the answers (they're right there ^) i just need to know HOW you find the enthalpy combustion of acetylene and ethanol.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which statement best describes how atoms combine to form sodium chloride (nacl)? a. a positively charged sodium ion and a positively charged chlorine ion form an covalent bond. b. a positively charged sodium ion and a negatively charged chlorine ion form an covalent bond. c. a positively charged sodium ion and a positively charged chlorine ion form an ionic bond. d. a positively charged sodium ion and a negatively charged chlorine ion form an ionic bond.
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3
You know the right answer?
Using the information in the table to the right, calculate the enthalpy of combustion of each of the...
Questions


Biology, 22.06.2019 20:30





Advanced Placement (AP), 22.06.2019 20:30




French, 22.06.2019 20:30


Chemistry, 22.06.2019 20:30

Health, 22.06.2019 20:30

Mathematics, 22.06.2019 20:30

Advanced Placement (AP), 22.06.2019 20:30

Mathematics, 22.06.2019 20:30


Geography, 22.06.2019 20:30

History, 22.06.2019 20:30



