
Chemistry, 10.12.2020 22:30 briannawilliams893
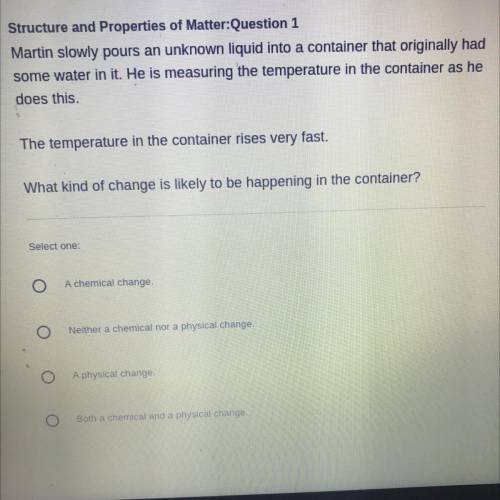
(HELP NEEDED) Martin slowly pours an unknown liquid into a container that originally had
some water in it. He is measuring the temperature in the container as he
does this.
The temperature in the container rises very fast.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
(HELP NEEDED) Martin slowly pours an unknown liquid into a container that originally had
some water...
Questions

Mathematics, 05.05.2020 02:29



Mathematics, 05.05.2020 02:29

Social Studies, 05.05.2020 02:29

English, 05.05.2020 02:29


Mathematics, 05.05.2020 02:29


English, 05.05.2020 02:29





Chemistry, 05.05.2020 02:29

Mathematics, 05.05.2020 02:29

Mathematics, 05.05.2020 02:29



Social Studies, 05.05.2020 02:29



