
Chemistry, 23.09.2019 05:20 ndkdidnfnk
Consider the formula for glucose: c6h12o6. what does this indicate about the relationship of the reactants to glucose?
a.) twelve water (h2o) molecules were used to make it.
b.) twelve hydrogen (h2) molecules were used to make it.
c.) six oxygen (o2) molecules were used to make it.
d.) six carbon dioxide (co2) molecules were used to make it.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Consider the formula for glucose: c6h12o6. what does this indicate about the relationship of the re...
Questions


Mathematics, 05.01.2021 06:30



English, 05.01.2021 06:30

History, 05.01.2021 06:30


Mathematics, 05.01.2021 06:30

History, 05.01.2021 06:30


Advanced Placement (AP), 05.01.2021 06:30

History, 05.01.2021 06:30





English, 05.01.2021 06:30

Mathematics, 05.01.2021 06:30

English, 05.01.2021 06:30

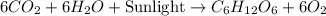
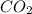 ) molecules were used to make it, indicate about the relationship of the reactants to glucose.
) molecules were used to make it, indicate about the relationship of the reactants to glucose.

