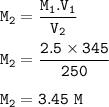Chemistry, 10.12.2020 19:00 mihirkantighosh
You have 345 mL of a 2.5 M NaCl solution. If you boil the water until the volume of the solution is 250 mLwhat will the molarity of the solution be?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
You have 345 mL of a 2.5 M NaCl solution. If you boil the water until the volume of the solution is...
Questions

Chemistry, 14.01.2021 18:10


Mathematics, 14.01.2021 18:10

Social Studies, 14.01.2021 18:10

Advanced Placement (AP), 14.01.2021 18:10




Chemistry, 14.01.2021 18:10

History, 14.01.2021 18:10

Biology, 14.01.2021 18:10

Mathematics, 14.01.2021 18:10

Mathematics, 14.01.2021 18:10


Mathematics, 14.01.2021 18:10


Mathematics, 14.01.2021 18:10


Mathematics, 14.01.2021 18:10

Chemistry, 14.01.2021 18:10