
Ammonia (A) diffuses through a stagnant layer of air (B), 1cm thick, at 25 ºC and 1 atm total pressure. The partial pressures of ammonia on the two sides of the air layer are: PA0=0.9 atm and PAl=0.1 atm respectively. Air is none diffusing. Calculate the molar flux of ammonia. DAB= 0.214 cm2 /s

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3


Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1
You know the right answer?
Ammonia (A) diffuses through a stagnant layer of air (B), 1cm thick, at 25 ºC and 1 atm total pressu...
Questions

English, 17.11.2020 05:10

Mathematics, 17.11.2020 05:10


Mathematics, 17.11.2020 05:10

Biology, 17.11.2020 05:10


History, 17.11.2020 05:10

Mathematics, 17.11.2020 05:10

Mathematics, 17.11.2020 05:10

History, 17.11.2020 05:10


Mathematics, 17.11.2020 05:10

History, 17.11.2020 05:10

History, 17.11.2020 05:10

Engineering, 17.11.2020 05:10


English, 17.11.2020 05:10



History, 17.11.2020 05:10

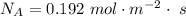
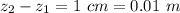
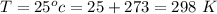
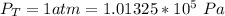
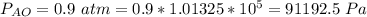
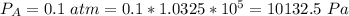
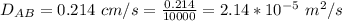
![N_A = \frac{D_{AB} * P_T }{RT(z_2 -z_1)} * ln [\frac{P_T - P_{Al}}{P_T - P_{AO}} ]](/tpl/images/0969/5009/086d8.png)
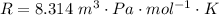
![N_A = \frac{2.14 *10^{-5} * 1.01325*10^{5} }{8.314 *298 (0.01)} * ln [\frac{1 - 0.1}{1 - 0.9} ]](/tpl/images/0969/5009/c7bba.png)


