
Chemistry, 10.12.2020 08:40 jademckinziemea
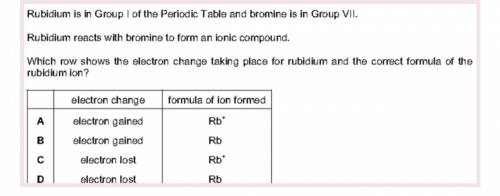
Rubidium is in Group I of the Periodic Table and bromine is in Group VII.
Rubidium reacts with bromine to form an ionic compound.
Which row shows the electron change taking place for rubidium and the correct formula of the
rubidium ion?
formula of ion formed
A
electron change
electron gained
electron gained
Rb
B
Rb
C
electron lost
Rb
D
electron lost
Rb

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1



You know the right answer?
Rubidium is in Group I of the Periodic Table and bromine is in Group VII.
Rubidium reacts with brom...
Questions



Mathematics, 19.09.2019 21:10


Mathematics, 19.09.2019 21:10

Biology, 19.09.2019 21:10




Mathematics, 19.09.2019 21:10

English, 19.09.2019 21:10




Physics, 19.09.2019 21:10


Geography, 19.09.2019 21:10


Social Studies, 19.09.2019 21:10



