Chemistry, 10.12.2020 06:20 jonathon3957
URGENT PLEASE HELP!
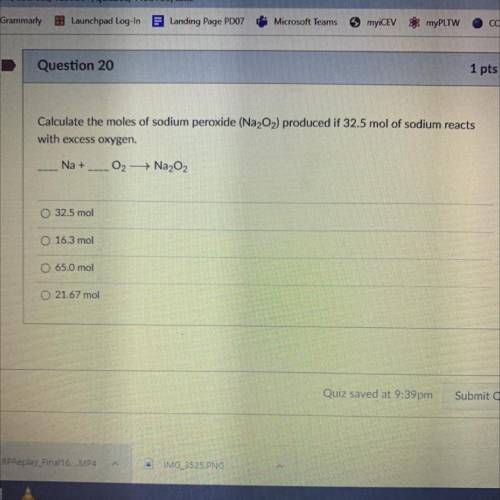
Calculate the moles of Sodium Peroxide (Na2O2) produced if 32.5 mol of sodium reacts with excess oxygen.
__Na+__O2—>Na2O2
A. 32.5 mol
B. 16.3 mol
C. 65.0 mol
D. 21.67 mol
and why is it one of the answer above?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
You know the right answer?
URGENT PLEASE HELP!
Calculate the moles of Sodium Peroxide (Na2O2) produced if 32.5 mol of sodium r...
Questions

Biology, 22.03.2021 09:20




Mathematics, 22.03.2021 09:20





Geography, 22.03.2021 09:20

English, 22.03.2021 09:20

Mathematics, 22.03.2021 09:20

Mathematics, 22.03.2021 09:20

Biology, 22.03.2021 09:20


Mathematics, 22.03.2021 09:20

Chemistry, 22.03.2021 09:20

Biology, 22.03.2021 09:20


Health, 22.03.2021 09:20