
Chemistry, 10.12.2020 04:00 sarahgilbert1468
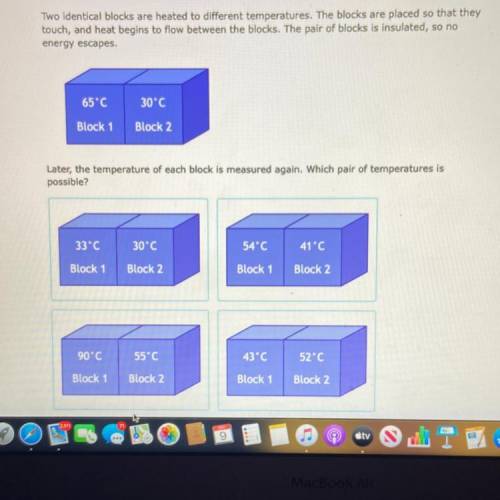
Two identical blocks are heated to different temperatures. The blocks are placed so that the
touch, and heat begins to flow between the blocks. The pair of blocks is insulated, so no
energy escapes.
65°C
Block 1
30*C
Block 2
Later, the temperature of each block is measured again. Which pair of temperatures is
possible?
41°C
33°C
Block 1
30°C
Block 2
54°C
Block 1
Blocs: 2
55°C
43°C
90°C
Block 1
52°C
Block 2
Block 2
Block 1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
Two identical blocks are heated to different temperatures. The blocks are placed so that the
touch,...
Questions

Mathematics, 06.01.2020 10:31

Engineering, 06.01.2020 10:31






Physics, 06.01.2020 10:31


Mathematics, 06.01.2020 10:31


Physics, 06.01.2020 10:31





History, 06.01.2020 10:31

Mathematics, 06.01.2020 10:31

Biology, 06.01.2020 10:31



