1. Write the equilibrium expression for each of the following reactions:
...

Chemistry, 10.12.2020 01:00 amselah735
1. Write the equilibrium expression for each of the following reactions:
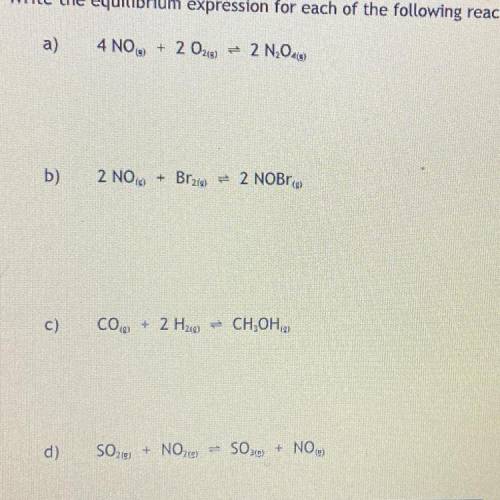

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 06:00
Which change will decrease the number of effective collisions during a chemical reaction? a. adding a catalyst b. increasing the surface area c. decreasing the temperature d. increasing the reactant concentrations e. increasing the volume of the reactants
Answers: 2

You know the right answer?
Questions

Mathematics, 07.08.2021 21:50



Chemistry, 07.08.2021 21:50



Mathematics, 07.08.2021 21:50



Biology, 07.08.2021 21:50

Mathematics, 07.08.2021 21:50


Mathematics, 07.08.2021 21:50

Business, 07.08.2021 21:50


Mathematics, 07.08.2021 22:00






