Ent will
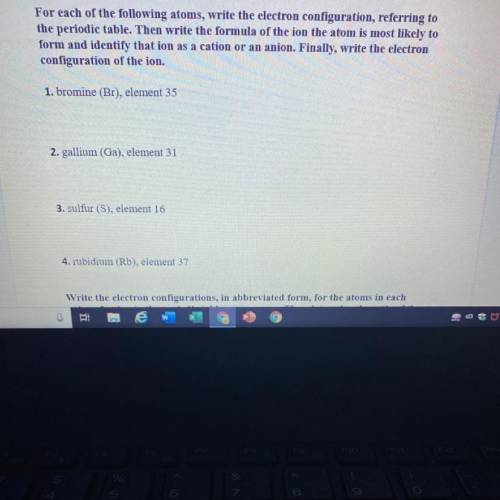
For each of the following atoms, write the electron configuration, referring to
the...

Chemistry, 10.12.2020 01:20 hebibova2016
Ent will
For each of the following atoms, write the electron configuration, referring to
the periodic table. Then write the formula of the ion the atom is most likely to
form and identify that ion as a cation or an anion. Finally, write the electron
configuration of the ion.
1. bromine (Br), element 35
2. gallium (Ga), element 31
3. sulfur (S), element 16
4. rubidium (Rb), element 37
Write the electron con
abbreviated

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Questions


Mathematics, 27.07.2021 17:00

Social Studies, 27.07.2021 17:00

Mathematics, 27.07.2021 17:00

Mathematics, 27.07.2021 17:00











English, 27.07.2021 17:00


Health, 27.07.2021 17:00


English, 27.07.2021 17:00



