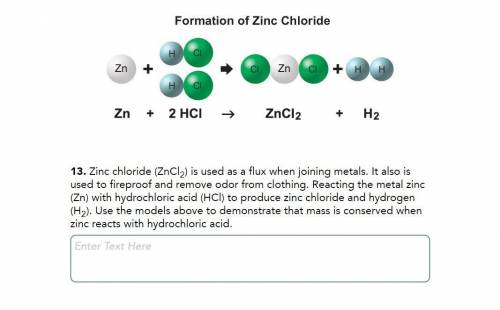
Zinc chloride (ZnCl2) is used as a flux when joining metals. It also is used to fireproof and remove odor from clothing. Reacting the metal zinc (Zn) with hydrochloric acid (HCl) to produce zinc chloride and hydrogen (H2). Use the models above to demonstrate that mass is conserved when zinc reacts with hydrochloric acid.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
You know the right answer?
Zinc chloride (ZnCl2) is used as a flux when joining metals. It also is used to fireproof and remove...
Questions

Mathematics, 06.11.2020 16:20

Mathematics, 06.11.2020 16:20











Biology, 06.11.2020 16:20

Mathematics, 06.11.2020 16:20