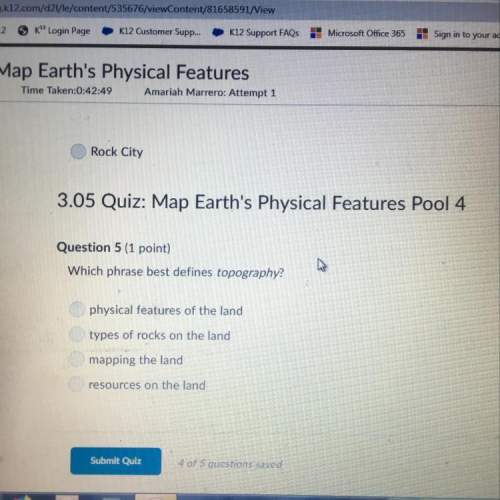
Chemistry, 10.12.2020 01:00 dakotacsey03
What is the net ionic equation for the reaction shown below? AgNO3(aq) + NaCl(aq) --> AgCl(s) + NaNO3(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1
You know the right answer?
What is the net ionic equation for the reaction shown below?
AgNO3(aq) + NaCl(aq) --> AgCl(s) +...
Questions






English, 06.06.2020 02:00



Mathematics, 06.06.2020 02:00


History, 06.06.2020 02:00


Mathematics, 06.06.2020 02:00


Mathematics, 06.06.2020 02:00


Mathematics, 06.06.2020 02:00

History, 06.06.2020 02:00