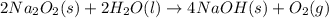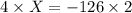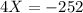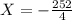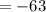Chemistry, 09.12.2020 22:50 cmflores3245
The value of ΔH° for the reaction below is -126 kJ. kj are released when 2.00 mol of NaOH is formed in the reaction?
2 Na2O2 (s) + 2 H2O (l) → 4NaOH (s) + O2 (g)
A) 252
B) 63
C) 3.9
D) 7.8
E) -126

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Select the correct answer from each drop-down menu. daniel and sanya are scientists. daniel is studying whether the increasing frequency of tropical storms is affecting coastal erosion. sanya is investigating whether the discharge from industrial plants has any impact on the ph concentration of freshwater swamps in the surrounding area. which fields of science are daniel’s and sanya’s studies most closely related to? daniel’s field of study is related to science, and sanya’s field of study is related to .
Answers: 3

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2
You know the right answer?
The value of ΔH° for the reaction below is -126 kJ. kj are released when 2.00 mol of NaOH is formed...
Questions

Physics, 20.10.2020 20:01


Mathematics, 20.10.2020 20:01


Mathematics, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

Chemistry, 20.10.2020 20:01

Biology, 20.10.2020 20:01



Mathematics, 20.10.2020 20:01



Mathematics, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01

Computers and Technology, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01


English, 20.10.2020 20:01