
Chemistry, 09.12.2020 23:50 hollicious1384
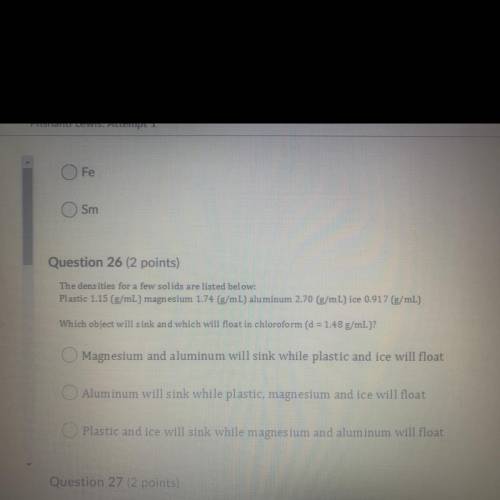
The densities for a few solids are listed below:
Plastic 1.15 (g/mL) magnesium 1.74 (g/mL) aluminum 2.70 (g/mL) ice 0.917 (g/mL)
Which object will sink and which will float in chloroform (d = 1.48 g/mL)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1
You know the right answer?
The densities for a few solids are listed below:
Plastic 1.15 (g/mL) magnesium 1.74 (g/mL) aluminum...
Questions





Biology, 01.11.2019 16:31

Mathematics, 01.11.2019 16:31



English, 01.11.2019 16:31

History, 01.11.2019 16:31


Mathematics, 01.11.2019 16:31

Mathematics, 01.11.2019 16:31




Social Studies, 01.11.2019 16:31


History, 01.11.2019 16:31



