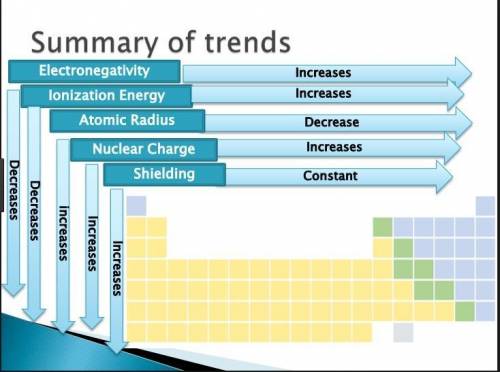
The Periodic Table of the Elements is useful for revealing patterns and trends in the elements. Which statement accurately describes a pattern in the size of atomic radii in the Periodic Table of the Elements?
*Atomic radii decrease from left to right across a period and decrease from top to
bottom in a group.
*Atomic radii increase from left to right across a period and increase from top to
bottom in a group.
*Atomic radii decrease from left to right across a period and increase from top to
bottom in a group.
*Atomic radii increase from left to right across a period and decrease from top to
Wottom in a group.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1


Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
The Periodic Table of the Elements is useful for revealing patterns and trends in the elements. Whic...
Questions





Advanced Placement (AP), 12.08.2020 05:01

English, 12.08.2020 05:01



English, 12.08.2020 05:01











Mathematics, 12.08.2020 05:01