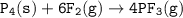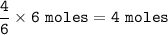Consider the following balanced equation:
P4(s) + 6F2(e)
4PF3(g)
If 1.25 moles of P4(s)...

Chemistry, 09.12.2020 17:00 tonydeanfbg8706
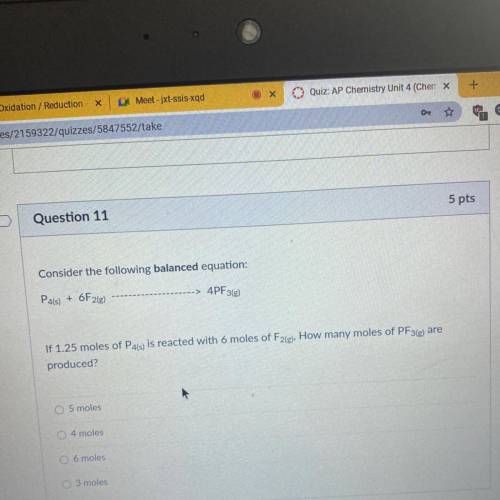
Consider the following balanced equation:
P4(s) + 6F2(e)
4PF3(g)
If 1.25 moles of P4(s) is reacted with 6 moles of F2(g), How many moles of PF3(e) are
produced?
O 5 moles
4 moles
O 6 moles
O 3 moles

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Questions

Social Studies, 21.04.2020 18:30





History, 21.04.2020 18:31


Mathematics, 21.04.2020 18:31


Mathematics, 21.04.2020 18:31


Mathematics, 21.04.2020 18:31



History, 21.04.2020 18:31





History, 21.04.2020 18:31