
Chemistry, 09.12.2020 06:40 stefaniethibodeaux
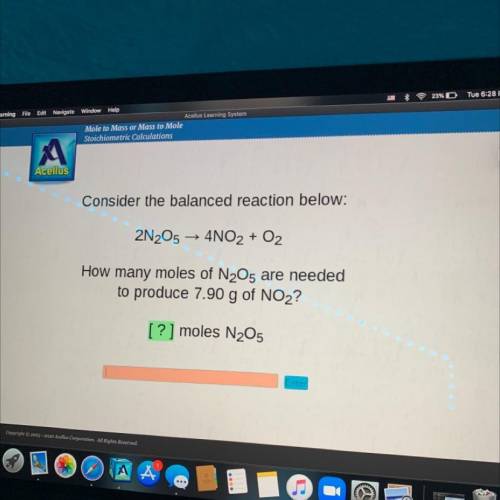
How many moles of N2O5 are needed
to produce 7.90 g of NO2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
How many moles of N2O5 are needed
to produce 7.90 g of NO2?
...
to produce 7.90 g of NO2?
...
Questions

Physics, 19.11.2020 17:30

English, 19.11.2020 17:30

History, 19.11.2020 17:30

Mathematics, 19.11.2020 17:30



Mathematics, 19.11.2020 17:30


Social Studies, 19.11.2020 17:30



History, 19.11.2020 17:30

Arts, 19.11.2020 17:30

Spanish, 19.11.2020 17:30



History, 19.11.2020 17:30





