
Chemistry, 08.12.2020 23:40 snowprincess99447
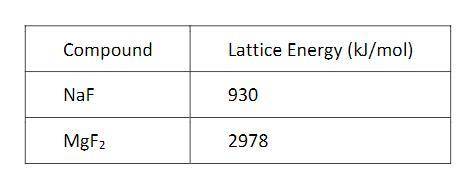
The energy required to dissociate an ionic solid into gaseous ions (lattice energy) for the compounds NaF and MgF2 are shown in the table above. On the basis of Coulomb’s law, which of the following best helps to explain the large difference between the lattice energies of NaF and MgF2?
a. The solubility of MgF2 is less than that of NaF.
b. The electronegativity of Mg is greater than that of Na.
c. The mass of the Mg cation is greater than that of the Na cation.
d. The charge of the Mg cation is larger than that of the Na cation.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

You know the right answer?
The energy required to dissociate an ionic solid into gaseous ions (lattice energy) for the compound...
Questions


Mathematics, 03.02.2021 22:00



Health, 03.02.2021 22:00

Mathematics, 03.02.2021 22:00


Mathematics, 03.02.2021 22:00



Mathematics, 03.02.2021 22:00


Mathematics, 03.02.2021 22:00

English, 03.02.2021 22:00


Health, 03.02.2021 22:00

English, 03.02.2021 22:00

Social Studies, 03.02.2021 22:00

Physics, 03.02.2021 22:00



