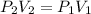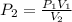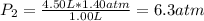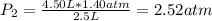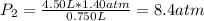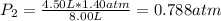Chemistry, 08.12.2020 16:50 itssergioa
The air in a 4.50 L tank has a pressure of 1.40 atm . What is the final pressure, in atmospheres, when the air is placed in tanks that have the following volumes, if there is no change in temperature and amount of gas?
a. 1.00 L
b. 2500. mL
c. 750. mL
d. 8.00 L

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 09:30
Large crystals are formed when igneous rocks cool very slowly igneous rocks cool very quickly sedimentary rock is eroded metamorphic rocks change into igneous rock
Answers: 1

Chemistry, 23.06.2019 10:30
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2
You know the right answer?
The air in a 4.50 L tank has a pressure of 1.40 atm . What is the final pressure, in atmospheres, wh...
Questions

Mathematics, 04.11.2019 20:31


Mathematics, 04.11.2019 21:31

Mathematics, 04.11.2019 21:31




Mathematics, 04.11.2019 21:31





Mathematics, 04.11.2019 21:31

Mathematics, 04.11.2019 21:31

Advanced Placement (AP), 04.11.2019 21:31