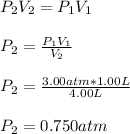A gas is initially confined to a 1.00 L vessel at 3.00 atm of pressure. A valve connecting the 1.00 L vessel to a 3.00 L vessel is opened and the gas expands. What is the final pressure in atm of the gas after the valve is opened, assuming that the volume of the connecting tube is negligible and there is no temperature change?
a. 0.250 atm
b. 0.500 atm
c. 0.750 atm
d. 1.00 atm
e. None of these choices

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

You know the right answer?
A gas is initially confined to a 1.00 L vessel at 3.00 atm of pressure. A valve connecting the 1.00...
Questions

Physics, 26.04.2021 22:30



Mathematics, 26.04.2021 22:30





World Languages, 26.04.2021 22:30

Mathematics, 26.04.2021 22:30


History, 26.04.2021 22:30


Mathematics, 26.04.2021 22:30



Physics, 26.04.2021 22:30

Mathematics, 26.04.2021 22:30

History, 26.04.2021 22:30

Mathematics, 26.04.2021 22:30