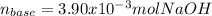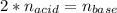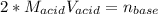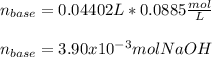Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
g How many moles of NaOH are present in a sample if it is titrated to its equivalence point with 44....
Questions

Mathematics, 12.08.2021 07:40


Mathematics, 12.08.2021 07:50

Mathematics, 12.08.2021 07:50

Mathematics, 12.08.2021 07:50


Mathematics, 12.08.2021 07:50

Mathematics, 12.08.2021 07:50

Mathematics, 12.08.2021 07:50

Mathematics, 12.08.2021 07:50

Social Studies, 12.08.2021 07:50


History, 12.08.2021 07:50

English, 12.08.2021 07:50


History, 12.08.2021 07:50

Mathematics, 12.08.2021 07:50


Mathematics, 12.08.2021 07:50