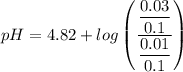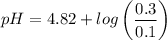Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1
You know the right answer?
Calculate the pH of a buffer when 0.010 moles of NaOH is added to 100. mL solution that is 0.20 M so...
Questions

Chemistry, 05.07.2019 22:00


History, 05.07.2019 22:00

Health, 05.07.2019 22:00



History, 05.07.2019 22:00





Social Studies, 05.07.2019 22:00



Geography, 05.07.2019 22:00

Mathematics, 05.07.2019 22:00



English, 05.07.2019 22:00

![pH = pKa + log \dfrac{[salt]}{[Acid]}](/tpl/images/0960/5001/27039.png)
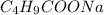 = 0.20 M
= 0.20 M 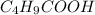 = 0.20 M
= 0.20 M