
Chemistry, 08.12.2020 05:50 potatogirl6811
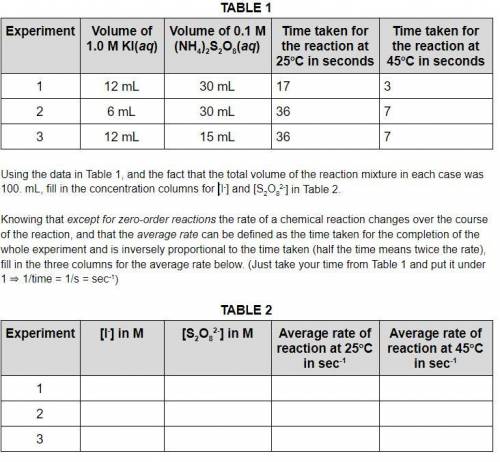
Using the data in Table 1, and the fact that the total volume of the reaction mixture in each case was 100. mL, fill in the concentration columns for [I-] and [S2O82-] in Table 2. Knowing that except for zero-order reactions the rate of a chemical reaction changes over the course of the reaction, and that the average rate can be defined as the time taken for the completion of the whole experiment and is inversely proportional to the time taken (half the time means twice the rate), fill in the three columns for the average rate below. (Just take your time from Table 1 and put it under 1 ⇒ 1/time = 1/s = sec-1)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1


Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
Using the data in Table 1, and the fact that the total volume of the reaction mixture in each case w...
Questions

History, 06.05.2020 20:20


History, 06.05.2020 20:20


Mathematics, 06.05.2020 20:20


History, 06.05.2020 20:20




History, 06.05.2020 20:20











