
Chemistry, 08.12.2020 05:20 alexusnicole817
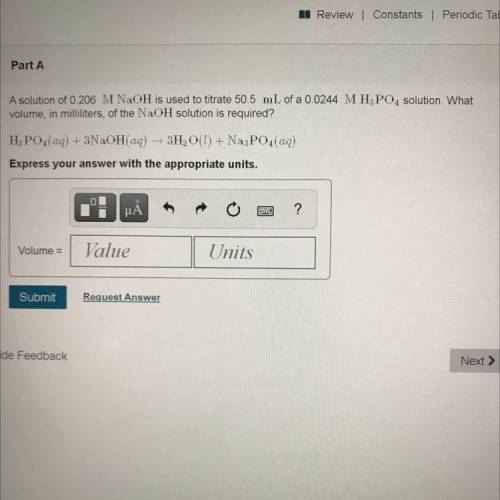
Part A
A solution of 0.206 M NaOH is used to titrate 50.5 mL of a 0.0244 MH3PO4 solution. What
volume, in milliliters, of the NaOH solution is required?
H3PO4(aq) + 3NaOH(aq) + 3H2O(l) + Na3PO4(aq)
Express your answer with the appropriate units.
μΑ
?
Volume =
Value
Units
Submit
Request Answer
Provide eedback

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Part A
A solution of 0.206 M NaOH is used to titrate 50.5 mL of a 0.0244 MH3PO4 solution. What
Questions

Physics, 28.01.2021 03:10

Chemistry, 28.01.2021 03:10




History, 28.01.2021 03:10


Mathematics, 28.01.2021 03:10

English, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10

English, 28.01.2021 03:10




Engineering, 28.01.2021 03:10

Business, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10

Mathematics, 28.01.2021 03:10



