
Chemistry, 08.12.2020 05:20 rainbowboi
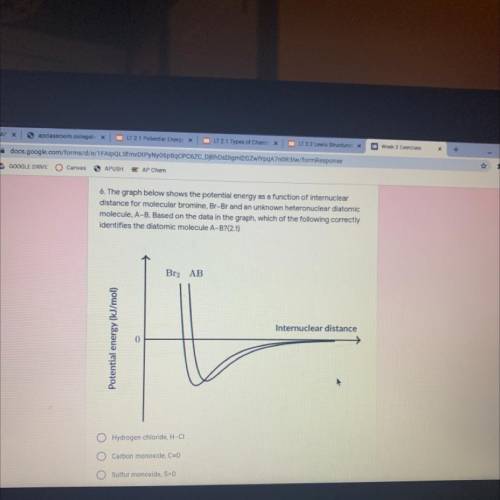
The graph below shows the potential energy as a function of internuclear
distance for molecular bromine, Br-Br and an unknown heteronuclear diatomic
molecule, A-B. Based on the data in the graph, which of the following correctly
identifies the diatomic molecule A-B?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
The graph below shows the potential energy as a function of internuclear
distance for molecular bro...
Questions



Mathematics, 27.07.2019 01:20

Mathematics, 27.07.2019 01:20

Mathematics, 27.07.2019 01:20

Mathematics, 27.07.2019 01:20

Mathematics, 27.07.2019 01:20


Mathematics, 27.07.2019 01:20

Arts, 27.07.2019 01:20












