
Chemistry, 08.12.2020 05:20 gonzalesrosalinda66
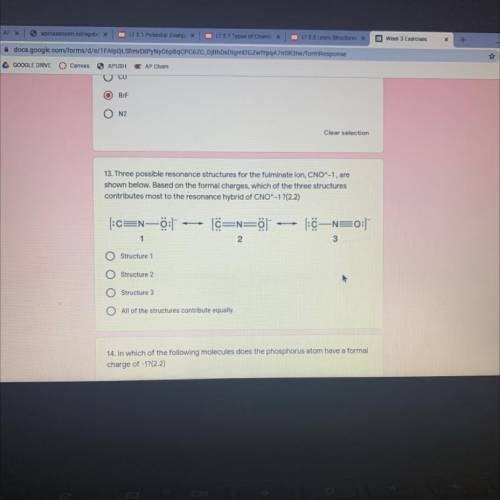
Three possible resonance structures for the fulminate ion, CNO^-1, are
shown below. Based on the formal charges, which of the three structures
contributes most to the resonance hybrid of CNO^-1 ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1
You know the right answer?
Three possible resonance structures for the fulminate ion, CNO^-1, are
shown below. Based on the fo...
Questions

English, 09.11.2020 14:00

History, 09.11.2020 14:00


Computers and Technology, 09.11.2020 14:00

Mathematics, 09.11.2020 14:00

Social Studies, 09.11.2020 14:00











Mathematics, 09.11.2020 14:00





