
Chemistry, 07.12.2020 14:00 shreyapatel2004
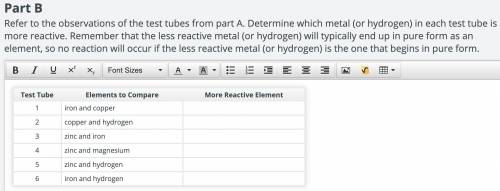
Refer to the observations of the test tubes from part A. Determine which metal (or hydrogen) in each test tube is more reactive. Remember that the less reactive metal (or hydrogen) will typically end up in pure form as an element, so no reaction will occur if the less reactive metal (or hydrogen) is the one that begins in pure form. (Attachment added! It's a Plato chart.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2


Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Refer to the observations of the test tubes from part A. Determine which metal (or hydrogen) in each...
Questions


Mathematics, 15.04.2020 01:08

Mathematics, 15.04.2020 01:08




Mathematics, 15.04.2020 01:08


Mathematics, 15.04.2020 01:09





Mathematics, 15.04.2020 01:09





History, 15.04.2020 01:09



