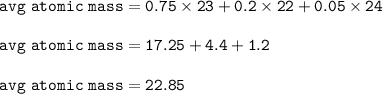Chemistry, 07.12.2020 06:30 myronpacis1128
A sample of sodium has a relative abundance of 75% with a mass number of 23, a relative abundance of 20% with a mass number of 22 and a relative abundance of 5% with a mass number of 24. What is the average atomic mass?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
A sample of sodium has a relative abundance of 75% with a mass number of 23, a relative abundance of...
Questions

Mathematics, 26.11.2019 02:31



Mathematics, 26.11.2019 02:31



Social Studies, 26.11.2019 02:31

English, 26.11.2019 02:31

Social Studies, 26.11.2019 02:31



Mathematics, 26.11.2019 02:31

Mathematics, 26.11.2019 02:31


Mathematics, 26.11.2019 02:31

Mathematics, 26.11.2019 02:31



Mathematics, 26.11.2019 02:31