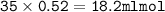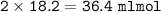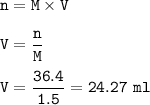Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1
You know the right answer?
HELP In a neutralization reaction, aqueous HCl and aqueous Ba(OH)2 react to form aqueous BaCl2 and w...
Questions




Mathematics, 01.09.2019 14:00


Mathematics, 01.09.2019 14:00

History, 01.09.2019 14:00



Mathematics, 01.09.2019 14:00

History, 01.09.2019 14:00

History, 01.09.2019 14:00



Social Studies, 01.09.2019 14:00

Mathematics, 01.09.2019 14:00

Mathematics, 01.09.2019 14:00

Mathematics, 01.09.2019 14:00