
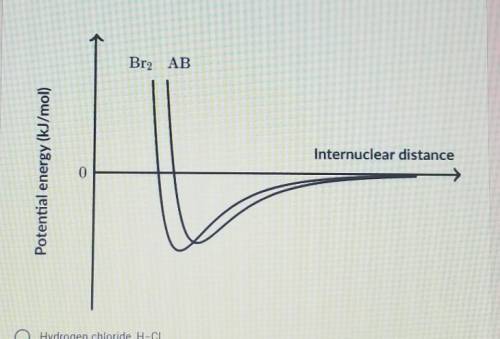
The graph below shows the potential energy as a function of internuclear distance for molecular bromine, Br-Br and an unknown heteronuclear diatomic molecule, A-B. Based on the data in the graph, which of the following correctly identifies the diatomic molecule A-B?
A.) Hydrogen Chloride, H-Cl
B.) Carbon Monoxide, C=_O (Triple Bond)
C.) Sulfur Monoxide, S=O (Double Bond)
D.) Iodine Monobromide, I-Br

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 03:30
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
The graph below shows the potential energy as a function of internuclear distance for molecular brom...
Questions

Mathematics, 12.02.2021 06:00


Mathematics, 12.02.2021 06:00

Mathematics, 12.02.2021 06:00


English, 12.02.2021 06:00

Mathematics, 12.02.2021 06:00




Mathematics, 12.02.2021 06:00

Mathematics, 12.02.2021 06:00

Chemistry, 12.02.2021 06:00

Mathematics, 12.02.2021 06:00

Chemistry, 12.02.2021 06:00

Mathematics, 12.02.2021 06:00


Biology, 12.02.2021 06:00

Mathematics, 12.02.2021 06:00

Biology, 12.02.2021 06:00



