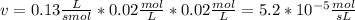Chemistry, 18.08.2019 12:00 emiliapizzillo
The rate law for a hypothetical reaction is rate = k [a][b]. if the concentrations of a and b are both 0.020 moles per liter and k = 1.3 × 10-1 m-1s-1, what is the reaction rate?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
The rate law for a hypothetical reaction is rate = k [a][b]. if the concentrations of a and b are bo...
Questions

Mathematics, 17.02.2020 18:02