
Chemistry, 05.12.2020 17:20 ghari112345
For each of the nuclei below write the isotope symbol. Explain how an atom of the unstable isotope carbon -14 becomes an atom of nitrogen 14 if the mass stays the same
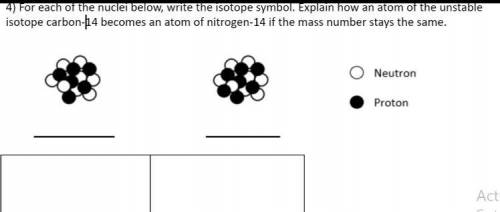

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 21:00
Two nails have identical sizes and shapes. in one nail, 20 percent of the domains are lined up. in the other nail, 80 percent of the domains are lined up. which has stronger magnetic force? first answer gets brainliest!
Answers: 1

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
For each of the nuclei below write the isotope symbol. Explain how an atom of the unstable isotope c...
Questions

Mathematics, 24.10.2019 03:00

Biology, 24.10.2019 03:00




Mathematics, 24.10.2019 03:00

History, 24.10.2019 03:00

Mathematics, 24.10.2019 03:00


Biology, 24.10.2019 03:00

Mathematics, 24.10.2019 03:00

History, 24.10.2019 03:00



Biology, 24.10.2019 03:00


Mathematics, 24.10.2019 03:00

History, 24.10.2019 03:00




