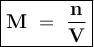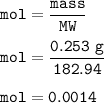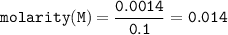Chemistry, 05.12.2020 03:10 Leggett8152
A chemist prepares a solution by adding 253 mg of Co(NO3)2 (MW = 182.94 g/mol ) to a volumetric flask, and then adding water until the total volume of the contents of the flask reaches the calibration line that indicates 100 mL . Determine the molarity of the prepared solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

You know the right answer?
A chemist prepares a solution by adding 253 mg of Co(NO3)2 (MW = 182.94 g/mol ) to a volumetric flas...
Questions



Mathematics, 21.05.2021 23:50

Mathematics, 21.05.2021 23:50

Mathematics, 21.05.2021 23:50


Mathematics, 21.05.2021 23:50

Biology, 21.05.2021 23:50

Advanced Placement (AP), 21.05.2021 23:50

Mathematics, 21.05.2021 23:50


Mathematics, 21.05.2021 23:50

Mathematics, 21.05.2021 23:50


Mathematics, 21.05.2021 23:50

Mathematics, 21.05.2021 23:50

Mathematics, 21.05.2021 23:50