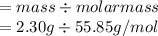Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
10. Given 2.30 grams of Fe, how many moles of FeCl3, can be produced? 2 Fe + 3 Cl2 → 2 FeCl3 A. 0.04...
Questions

English, 19.05.2020 14:59


History, 19.05.2020 14:59

Mathematics, 19.05.2020 14:59

Mathematics, 19.05.2020 14:59

Mathematics, 19.05.2020 14:59


Law, 19.05.2020 14:59



Mathematics, 19.05.2020 14:59

History, 19.05.2020 14:59

Mathematics, 19.05.2020 14:59



Mathematics, 19.05.2020 14:59


History, 19.05.2020 14:59