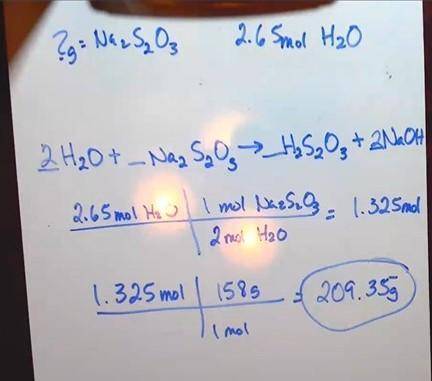Chemistry, 04.12.2020 22:10 aljalloh94
PLS HELP! I CAN"T DO THIS! I AM ABOUT TO CRY COZ NO ONE WILL ANSWER TnT
Chlorine is used by textile manufacturers to bleach cloth. Excess chlorine is destroyed by its reaction with sodium thiosulfate, Na 2 S 2 O 3 :
Na 2 S 2 O 3(aq) + 4Cl 2(g) + 5H 2 O (aq) 2NaHSO 4(aq) + 8HCl (aq)
1. How many grams of Na 2 S 2 O 3 are needed to react with 2.65 mol of H 2 O? (3
marks)
2. How many mol of HCl can form from 25.2 mol of Na 2 S 2 O 3 ? (2 marks)
3. How many Liters of Cl 2 are required to produce 15.7 moles of NaHSO 4 ? (2
marks)
4. How many molecules of HCl can form from 4.92 grams of H 2 O? (3 marks)
I also need the steps. Please and thank you!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1


Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
PLS HELP! I CAN"T DO THIS! I AM ABOUT TO CRY COZ NO ONE WILL ANSWER TnT
Chlorine is used by textile...
Questions

Social Studies, 21.01.2021 22:00

Computers and Technology, 21.01.2021 22:00

Computers and Technology, 21.01.2021 22:00


Health, 21.01.2021 22:00

English, 21.01.2021 22:00






History, 21.01.2021 22:00

Computers and Technology, 21.01.2021 22:00

Mathematics, 21.01.2021 22:00


English, 21.01.2021 22:00

Health, 21.01.2021 22:00

Social Studies, 21.01.2021 22:00

History, 21.01.2021 22:00