
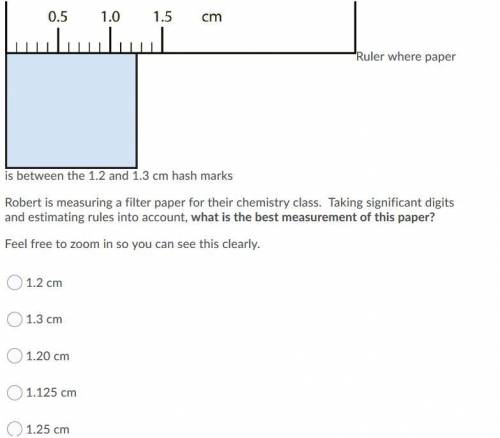
Robert is measuring a filter paper for their chemistry class. Taking significant digits and estimating rules into account, what is the best measurement of this paper? Feel free to zoom in so you can see this clearly.
A. 1.2 cm
B. 1.3 cm
C. 1.20 cm
D. 1.125 cm
E. 1.25 cm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Robert is measuring a filter paper for their chemistry class. Taking significant digits and estimati...
Questions


Physics, 25.03.2020 22:28




History, 25.03.2020 22:28

English, 25.03.2020 22:28




Mathematics, 25.03.2020 22:28



Geography, 25.03.2020 22:29


Mathematics, 25.03.2020 22:29




English, 25.03.2020 22:29



