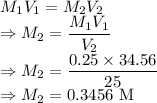Chemistry, 04.12.2020 01:00 haleyscales825
A solution of HCl with a volume of 25.00 mL is titrated to the endpoint, with 0.250 M
NaOH. If it takes 34.56 mL of NaOH, what is the original concentration of HCl in the
solution?
HCl(aq) + NaOH(aq) → H20(l)+ NaCl(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
A solution of HCl with a volume of 25.00 mL is titrated to the endpoint, with 0.250 M
NaOH. If it t...
Questions


Mathematics, 19.05.2021 07:10


Computers and Technology, 19.05.2021 07:10


Mathematics, 19.05.2021 07:10

Mathematics, 19.05.2021 07:10

Social Studies, 19.05.2021 07:10


English, 19.05.2021 07:10


Mathematics, 19.05.2021 07:10



Mathematics, 19.05.2021 07:20

Mathematics, 19.05.2021 07:20


Health, 19.05.2021 07:20

Mathematics, 19.05.2021 07:20

Mathematics, 19.05.2021 07:20

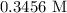
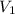 = Volume of NaOH = 34.56 mL
= Volume of NaOH = 34.56 mL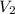 = Volume of HCl = 25 mL
= Volume of HCl = 25 mL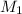 = Concentration of NaOH = 0.25 M
= Concentration of NaOH = 0.25 M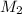 = Concentration of HCl
= Concentration of HCl