
Chemistry, 03.12.2020 22:00 GEEKLIFE6598
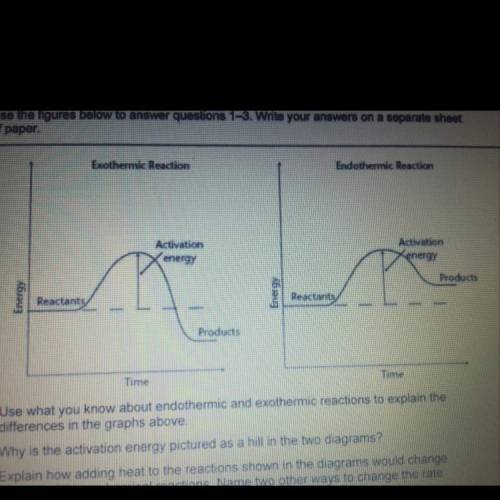
Use what you know about endothermic and exothermic reactions to explain the differences in the graphs above

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1


Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

You know the right answer?
Use what you know about endothermic and exothermic reactions to explain the differences in the graph...
Questions

Mathematics, 26.08.2019 03:30






History, 26.08.2019 03:30


Geography, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30

History, 26.08.2019 03:30


Mathematics, 26.08.2019 03:30


Chemistry, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30

History, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30

Mathematics, 26.08.2019 03:30



