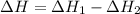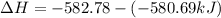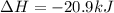Chemistry, 03.12.2020 20:10 haileywebb8
The phase change between white tin and gray tin is difficult to observe directly. Both substances can be burned, however. From these equations, calculate AHⓇ for the conversion of gray tin into white tin:
Sn(s, white) + O2(g) + SnO2(g) AH = -580.69 kJ
Sn(s, gray) + O2(g) + SnO2(g) AH° = -582.78 kJ
AH = kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
The phase change between white tin and gray tin is difficult to observe directly. Both substances ca...
Questions


Mathematics, 09.03.2021 18:50


Social Studies, 09.03.2021 18:50

Health, 09.03.2021 18:50



Mathematics, 09.03.2021 18:50




Mathematics, 09.03.2021 18:50

History, 09.03.2021 18:50


Mathematics, 09.03.2021 18:50

Spanish, 09.03.2021 18:50


Chemistry, 09.03.2021 18:50


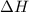 for the conversion of gray tin to white tin is -20.9 kJ
for the conversion of gray tin to white tin is -20.9 kJ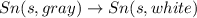
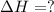
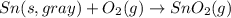
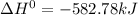 (1)
(1)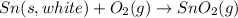
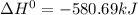 (2)
(2)