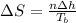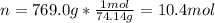Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
The heat of vaporization of diethyl ether is . Calculate the change in entropy when of diethyl ether...
Questions

Mathematics, 17.10.2020 01:01

Chemistry, 17.10.2020 01:01

Computers and Technology, 17.10.2020 01:01

English, 17.10.2020 01:01



Mathematics, 17.10.2020 01:01


Mathematics, 17.10.2020 01:01



Mathematics, 17.10.2020 01:01

Chemistry, 17.10.2020 01:01

English, 17.10.2020 01:01


Mathematics, 17.10.2020 01:01


History, 17.10.2020 01:01