
Chemistry, 03.12.2020 18:10 fernandaElizondo
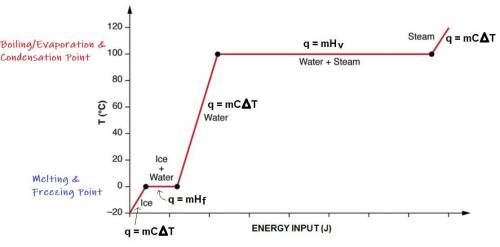
A 200 g sample of water at 60.0 degrees Celsius is heated to water vapor at 140.0 degrees Celsius. Expected Answer = 501,440 J Before trying to solve this problem, explain:
what is happening to the water from 60.0 degrees Celsius to 100.0 degrees Celsius?
what happens at 100.0 degrees Celsius?
what happens from 100.0 degrees Celsius to 140.0 degrees Celsius?
Then solve the full problem, showing work & units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
A 200 g sample of water at 60.0 degrees Celsius is heated to water vapor at 140.0 degrees Celsius. E...
Questions

Chemistry, 21.06.2019 19:30

English, 21.06.2019 19:30

Computers and Technology, 21.06.2019 19:30





Mathematics, 21.06.2019 19:30


Business, 21.06.2019 19:30

Chemistry, 21.06.2019 19:30

Chemistry, 21.06.2019 19:30



Mathematics, 21.06.2019 19:30

Mathematics, 21.06.2019 19:30

Biology, 21.06.2019 19:30

Chemistry, 21.06.2019 19:30




