Chemistry, 03.12.2020 17:00 jasminemmathlover
Part C
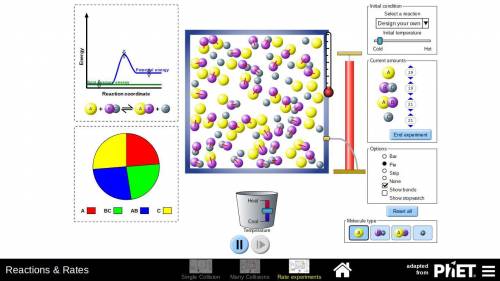
In the Options window of the simulation, select Pie to show the pie chart. In the window labeled Start with how many, use the up arrows to add 40 particles of A and 40 particles of BC. Then press the Begin experiment button. Wait a few seconds for the reaction to come to equilibrium, and then click pause near the bottom of the screen.
Study the graph on the left. The green line shows total average energy. The blue line shows the amount of potential energy possessed by the reactants and the products. Recall that substances most readily take the form in which they have the least potential energy. The amount of potential energy can be adjusted, affecting the outcome of the reaction. Recall that in the initial experiment for synthesizing ammonia, only about 20% of the reactants were converted to the products.
Click the Play arrow to resume the simulation. Then adjust the potential energy curve so the proportion of reactants to products is roughly the same as the proportion in the initial experiment. Adjust the center slider up or down so there’s a bump in the middle of the energy curve. The bump represents the energy the particles must have to react. Take a screenshot of the energy curve, and use the Insert Image button to paste it in the answer space.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
Part C
In the Options window of the simulation, select Pie to show the pie chart. In the window lab...
Questions

Mathematics, 30.06.2019 09:00




Mathematics, 30.06.2019 09:00


Mathematics, 30.06.2019 09:00

History, 30.06.2019 09:00

History, 30.06.2019 09:00


Biology, 30.06.2019 09:00

Mathematics, 30.06.2019 09:00

Mathematics, 30.06.2019 09:00

History, 30.06.2019 09:00

Mathematics, 30.06.2019 09:00

Health, 30.06.2019 09:00



Mathematics, 30.06.2019 09:00

Mathematics, 30.06.2019 09:00