Chemistry, 03.12.2020 07:00 alexreddin3127
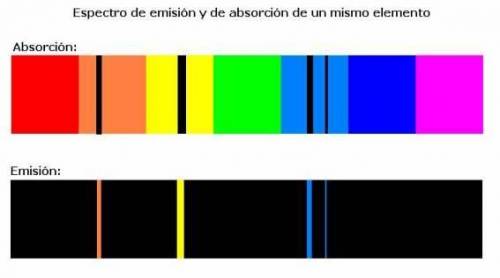
What does an atomic emission spectrum look like if the electrons energy levels in an atom were not quantizied
a. lines would be shifted into the ultraviole region
b. there would be fewer lines
c. there will be more lines
d. the spectrum would be constnuous

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

You know the right answer?
What does an atomic emission spectrum look like if the electrons energy levels in an atom were not q...
Questions





Mathematics, 12.06.2021 01:00

History, 12.06.2021 01:00

Mathematics, 12.06.2021 01:00


History, 12.06.2021 01:10


Health, 12.06.2021 01:10

History, 12.06.2021 01:10


Mathematics, 12.06.2021 01:10


Mathematics, 12.06.2021 01:10

Social Studies, 12.06.2021 01:10

Spanish, 12.06.2021 01:10

Mathematics, 12.06.2021 01:10

Chemistry, 12.06.2021 01:10