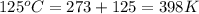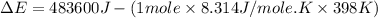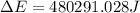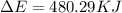Chemistry, 31.08.2019 00:00 nathaniel12
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are converted h2(g) and o2(g) against a pressure of 1 atm at 125 degrees celcius what is δe of reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
Consider the reaction: 2 h2o (> 2 h2 (g) + o2 (g). δh=483.6 kj/mol. if 2 moles of h2o (g) are co...
Questions

Mathematics, 14.01.2021 03:20

English, 14.01.2021 03:20


English, 14.01.2021 03:20

Mathematics, 14.01.2021 03:20

Physics, 14.01.2021 03:20


Mathematics, 14.01.2021 03:20

Mathematics, 14.01.2021 03:20

English, 14.01.2021 03:20


Mathematics, 14.01.2021 03:20

Mathematics, 14.01.2021 03:20

English, 14.01.2021 03:20

Mathematics, 14.01.2021 03:20


Mathematics, 14.01.2021 03:20

Health, 14.01.2021 03:20


Arts, 14.01.2021 03:20

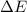 of the reaction is, 480.29 KJ.
of the reaction is, 480.29 KJ.
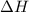 = enthalpy of the reaction = 483.6 KJ/mole = 483600 J
= enthalpy of the reaction = 483.6 KJ/mole = 483600 J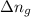 = change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole
= change in the moles of the reaction = Moles of product - Moles of reactant = 3 - 2 = 1 mole