
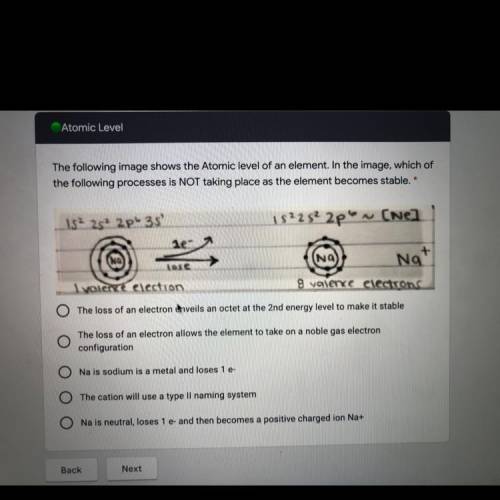
The following image shows the Atomic level of an element. In the image, which of
the following processes is NOT taking place as the element becomes stable. *
152 25+ 2p 35
152252 2p on ~ (Nel
le-
Na
+
Nat"
Tose
Iyolence election
8 valence electrons
O The loss of an electron Anveils an octet at the 2nd energy level to make it stable
The loss of an electron allows the element to take on a noble gas electron
configuration
Na is sodium is a metal and loses 1 e-
O The cation will use a type Ii naming system
O Na is neutral, loses 1 e- and then becomes a positive charged ion Na+

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Consider three unlabeled bottles, each contain small pieces of one of the following metals. - magnesium - sodium - silver the following reagents are used for identifying the metals. - pure water - a solution of 1.0 molar hcl - a solution of concentrated hno3 (a) which metal can be easily identified because it is much softer than the other two? describe a chemical test that distinguishes this metal from the other two, using only one of the reagents above. write a balanced chemical equation for the reaction that occurs. (b) one of the other two metals reacts readily with the hcl solution. identify the metal and write the balanced chemical equation for the reaction that occurs when this metal is added to the hcl solution. use the table of standard reduction potentials (attached) to account for the fact that this metal reacts with hcl while the other does not. (c) the one remaining metal reacts with the concentrated hno3 solution. write a balanced chemical equation for the reaction that occurs. (d) the solution obtained in (c) is diluted and a few drops of 1 m hcl is added. describe what would be observed. write a balanced chemical equation for the reaction that occurs.
Answers: 2

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1
You know the right answer?
The following image shows the Atomic level of an element. In the image, which of
the following proc...
Questions

Mathematics, 15.08.2021 14:00





Mathematics, 15.08.2021 14:00

Chemistry, 15.08.2021 14:00



Mathematics, 15.08.2021 14:00



History, 15.08.2021 14:00

History, 15.08.2021 14:00



Mathematics, 15.08.2021 14:00

Biology, 15.08.2021 14:00

Arts, 15.08.2021 14:00

English, 15.08.2021 14:00



