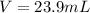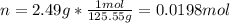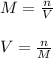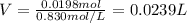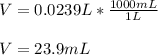Chemistry, 02.12.2020 14:00 isabelperez063
1. If 2.49 g of CuNO3 is dissolved in water to make a 0.830 M solution, what is the volume of the solution in milliliters?
2. How many moles of NaOH are present in 13.5 mL of 0.170 M NaOH?
3. Calculate the molarity of 0.650 mol of Na2S in 1.15 L of solution.
4. A student in lab needs to make a solution that is 7.00% by mass NaCl. If 145 g of NaCl is available, what mass of solution can be prepared?
5. Calculate the molarity of 24.1 g of MgS in 777 mL of solution.
6. A solution made by adding 16.3mL of methyl alcohol to enough water to give 541
mL of solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 04:00
Actual ingredients of lab (the cookies i am actually making) 1/2 cup sugar 1/2 cup brown sugar 1 1/3 stick margarine 1 egg 1/2 tsp salt 1 tsp vanilla 1/2 tsp baking soda 1 1/2 cup flour 1 1/3 cup chocolate chip can you answer the questions below ? discussion 1. suppose you are given the following amounts of ingredients: 1 dozen eggs 24 tsp. of vanilla 1 lb. (82 tsp.) of salt 1 lb. (84 tsp.) of baking soda 3 cups of chocolate chips 5 lb. (11 cups) of sugar 2 lb. (4 cups) of brown sugar 1 lb. (4 sticks) of margarine a. for each ingredient, calculate how many cookies could be prepared if all of that ingredient were consumed. (for example, the recipe shows that using 1 egg- with the right amounts of the other ingredients- yields 24 cookies. how many cookies can you make if the recipe is increased proportionately for 12 eggs? ) b. to determine the limiting reactant for the new ingredients list, identify which ingredient will result in the fewest number of cookies. c. what is the maximum number of cookies that can be produced from the new amounts of ingredients?
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
1. If 2.49 g of CuNO3 is dissolved in water to make a 0.830 M solution, what is the volume of the so...
Questions

Mathematics, 27.08.2019 02:30

Biology, 27.08.2019 02:30




Spanish, 27.08.2019 02:30

Physics, 27.08.2019 02:30


Biology, 27.08.2019 02:30

Social Studies, 27.08.2019 02:30

French, 27.08.2019 02:30

Social Studies, 27.08.2019 02:30

Chemistry, 27.08.2019 02:30


Mathematics, 27.08.2019 02:30


Health, 27.08.2019 02:30

Mathematics, 27.08.2019 02:30